Abstract
Background: Haploidentical blood and marrow transplantation (BMT) substantially expands the pool of potential hematopoietic stem cell donors, including for minority groups. However, the outcomes of haploidentical transplantation remain inferior to matched unrelated donor (MUD) transplants when a standard post-transplant cyclophosphamide (PTCy)-based graft versus host disease (GvHD) prophylaxis regimen is employed in both settings. To address this issue, we sought to develop a more effective GvHD prevention regimen in haploidentical transplantation. While abatacept (Aba) seems to decrease the incidence of acute GvHD, PTCy is particularly effective in the prevention of chronic GvHD. Therefore, we designed a regimen that combines the merits of both agents and examined whether such approach can allow early withdrawal of tacrolimus (Tac).
Methods: We enrolled 46 adult patients with hematological malignancies receiving haploidentical transplantation from a relative in a prospective single center pilot phase Ib-II clinical trial. Patients received non-myeloablative, reduced-intensity or myeloablative preparative regimens followed by G-CSF mobilized peripheral blood transplants and a combination of PTCy (50mg/kg on days +3 and +4), Aba (10mg/kg on days +5, +14 and +28) and Tac at a dose adjusted based on blood level. Tac taper was initiated on day +60 and completed by day +90. After treating 30 patients, the study was amended to administer an additional dose of Aba on day +56 based on the noted timing of acute GvHD development and Aba terminal ½ life. Patients received standard supportive care by institutional practice including G-CSF and anti-microbial prophylaxis. Serologically CMV positive patients also received letermovir prophylaxis.
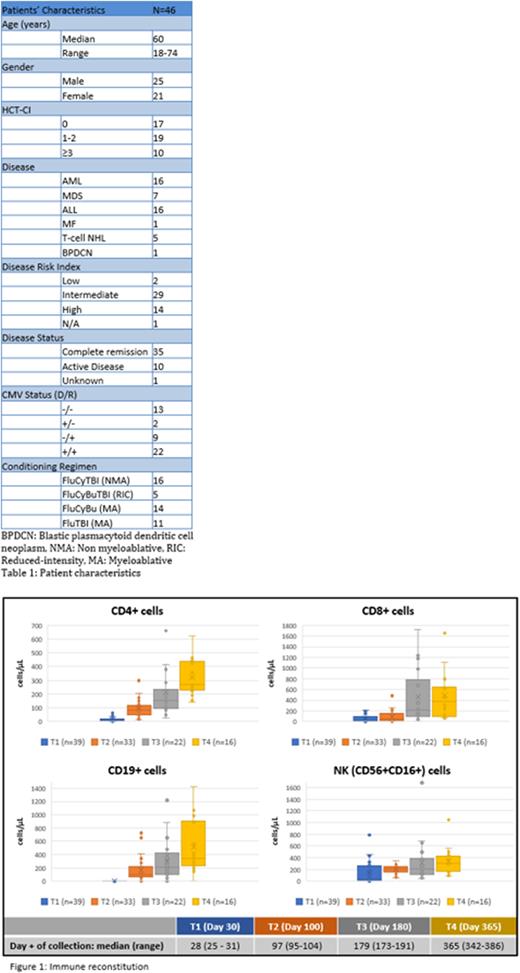
Results: Patient characteristics are summarized in table 1. Importantly, 19 out of 46 (41.3%) patients were from racial or ethnic minorities. The data cutoff date was July 29th, 2022. Three patients were excluded from this analysis due to short follow-up. For the remaining patients, median follow-up was 8.9 months. Median time to neutrophil engraftment was 18 days (13-30). One patient died before achieving platelet (plt) engraftment and 2 patients have not yet achieved plt engraftment. For the remaining 40 patients, median time to plt engraftment was 29 days (16-52). All patients achieved full donor chimerism at the time of count recovery. Tac was successfully tapered off as planned in all but 5 patients. Cumulative incidence of acute GvHD grades II-IV, III-IV and IV with death as competing event was 12.8%, 5.1% and 0%, respectively. One-year (y) cumulative incidence of moderate to severe chronic GvHD was 17.1%. All cases of acute GvHD except 1 grade II acute GvHD were observed in the first 30 patients. Cases of chronic GvHD included 2 cases of overlap syndrome. There was no case of steroid-refractory acute GvHD or GvHD-related death. One-y cumulative incidence of relapse (non-relapse mortality as competing event) was 8.3% (95% CI 0%-17.1%). KM estimates of 1-year relapse-free survival (RFS), overall survival (OS) and GRFS (composite end point of grade III-IV acute GvHD-, chronic GvHD requiring systemic therapy- and relapse-free survival) were 91.7% (95% CI 83%-100%), 90.6% (95% CI 80.9%-100%) and 71.8% (95% CI 57.2%-90%), respectively. None of the safety stopping rules was triggered. There was 1 case of thrombotic microangiopathy due to Tac and 2 cases of sinusoidal occlusive disease, 1 requiring treatment with defibrotide; all 3 patients recovered. There were 2 cases of respiratory failure of undetermined etiology. One-y cumulative incidence of non-relapse mortality (relapse as competing event) was 2.6% (95% CI 0%-7.8%). The incidence of CMV and EBV reactivation was 39.5% and 2.3%, respectively. There was no case of adenovirus or HHV-6 virus reactivation. BK viruria occurred in 27.9% of patients with limited symptomatology (1 patient required treatment with cidofovir). Immune reconstitution results are summarized in figure 1.
Conclusion: CAST is a new GvHD prevention regimen that seems safe and effective, yielding excellent outcomes. CAST also allows early discontinuation of Tac. Outcomes with longer follow-up will be presented at the meeting. If the results are confirmed, CAST might be a step forward toward closing an important health disparity gap in BMT, equalizing the outcomes of haploidentical transplantation and MUD transplants.
Disclosures
Al-Homsi:BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees. Abdul-Hay:Jazz: Consultancy, Speakers Bureau; Servier: Speakers Bureau; Takeda: Speakers Bureau.
OffLabel Disclosure:
Cyclophosphamide for GvHD prevention Abatacept for GvHD prevention in haploidentical transplantation Tacrolimus for GvHD prevention
Author notes
Asterisk with author names denotes non-ASH members.